|
| |
|
|
|
|
|

NanothermiteThis page is to discuss the scientific facts about thermite and nanothermite. We also discuss how these materials related to very specific False Flag Black Operations conducted by elite forces within the United States Government on 9/11/2001
Nanothermite was first developed at Los Alamos National Laboratories, under the supervision and security of the same Bush Connection CIA front company which had security contracts with the Port Authority of NY to install "Security Upgrades" inside the WTC leading up to 9/11. Linear shaped Nanothermite demolition charged were developed in secret by the Komatsu-Dresser Mining Division. L. Paul Bremer who sat on that companies' board of directors went on to become a manager at Marsh and Mclennon and had an office in the WTC's south tower. These factors combined solidify nanothermite as a key piece of the 9/11 puzzle. Other demolition theories or debunkers who try to turn the 9/11 Truth Movement away from research into nanothermite as effectively working to undermine the Truth Movement and lead them in the wrong direction, away from the vital informatio outlined in the above two paragraphs. See "9/11 Suspects: Explosive Connections" video on YouTube. Nanothermite (from wikipedia)Nano-thermite, also called "super-thermite",[1] is the common name for a subset of metastable intermolecular composites (MICs) characterized by a highly exothermic reaction after ignition. Nano-thermites contain an oxidizer and a reducing agent, which are intimately mixed on the nanometer scale. MICs, including nano-thermitic materials, are a type of reactive materials investigated for military use, as well as in applications in propellants, explosives, and pyrotechnics. What separates MICs from traditional thermites is that the oxidizer and a reducing agent, normally iron oxide and aluminium are not a fine powder, but rather nanoparticles. This dramatically increases the reactivity relative to micrometre-sized powder thermite. As the mass transport mechanisms that slow down the burning rates of traditional thermites are not so important at these scales, the reactions become kinetically controlled and much faster. UsesHistorically, pyrotechnic or explosive applications for traditional thermites have been limited due to their relatively slow energy release rates. But because nanothermites are created from reactant particles with proximities approaching the atomic scale, energy release rates are far improved.[2] MICs or Super-thermites are generally developed for military use, propellants, explosives, and pyrotechnics. Because of their highly increased reaction rate, nanosized thermitic materials are being researched by the U.S. military with the aim of developing new types of bombs that are several times more powerful than conventional explosives.[3] Nanoenergetic materials can store higher amounts of energy than conventional energetic materials and can be used in innovative ways to tailor the release of this energy. Thermobaric weapons are considered to be a promising application of nanoenergetic materials. Research into military applications of nano-sized materials began in the early 1990s.[4] TypesThere are many possible thermodynamically stable fuel-oxidizer combinations. Some of them are:
In military research, aluminium-molybdenum oxide, aluminium-Teflon and aluminium-copper(II) oxide have received considerable attention.[4] Other compositions tested were based on nanosized RDX and with thermoplastic elastomers. PTFE or other fluoropolymer can be used as a binder for the composition. Its reaction with the aluminium, similar to magnesium/teflon/viton thermite, adds energy to the reaction. [5] Of the listed compositions, the Al-KMnO4 one shows the highest pressurization rates, followed by orders of magnitude slower Al-MoO3 and Al-CuO, followed by yet slower Al-Fe2O3. [6] Nanoparticles can be prepared by spray drying from a solution, or in case of insoluble oxides, spray pyrolysis of solutions of suitable precursors. The composite materials can be prepared by sol-gel techniques or by conventional wet mixing and pressing. Similar but not identical systems are nano-laminated pyrotechnic compositions, or energetic nanocomposites. In these systems, the fuel and oxidizer is not mixed as small particles, but deposited as alternating thin layers. For example, an energetic multilayer structure may be coated with an energetic booster material. Through selection of materials (the range of which includes virtually all metals) and size scale of the layers, functional properties of the multilayer structures can be controlled, such as the reaction front velocity, the reaction initiation temperature, and the amount of energy delivered by a reaction of alternating unreacted layers of the multilayer structure.[7] ProductionA method for producing nanoscale, or ultra fine grain (UFG) aluminum powders, a key component of most nano-thermitic materials, is the dynamic gas-phase condensation method, pioneered by Wayne Danen and Steve Son at Los Alamos National Laboratory. A variant of the method is being used at the Indian Head Division of the Naval Surface Warfare Center. Another production method for nanoaluminum powder is the pulsed plasma process developed by NovaCentrix (formerly Nanotechnologies).[8] The powders made by both processes are indistinguishable.[9] A critical aspect of the production is the ability to produce particles of sizes in the tens of nanometer range, as well as with a limited distribution of particle sizes. In 2002, the production of nano-sized aluminum particles required considerable effort, and commercial sources for the material were limited.[4] Current production levels are now beyond 100 kg/month. An application of the sol-gel method, developed by Randall Simpson, Alexander Gash and others at the Lawrence Livermore National Laboratory, can be used to make the actual mixtures of nanostructured composite energetic materials. Depending on the process, MICs of different density can be produced. Highly porous and uniform products can be achieved by supercritical extraction.[4] IgnitionNanoscale composites are easier to ignite than traditional thermites. A nichrome bridgewire can be used in some cases. Other means of ignition can include flame or laser pulse. Los Alamos National Laboratory (LANL) is developing super-thermite electric matches that use comparatively low ignition currents and resist friction, impact, heat and static discharge.[1] MICs have been investigated as a possible replacement for lead (e.g. lead styphnate, lead azide) containing percussion caps and electric matches. Compositions based on Al-Bi2O3 tend to be used. PETN may be optionally added.[10][11] MICs can be also added to high explosives to modify their properties. [12] Aluminium is typically added to explosives to increase their energy yield. Addition of small amount of MIC to aluminium powder increases overall combustion rate, acting as a burn rate modifier.[13] The products of a thermite reaction, resulting from ignition of the thermitic mixture, are usually metal oxides and elemental metals. At the temperatures prevailing during the reaction, the products can be solid, liquid or gaseous, depending on the components of the mixture.[14] Super-thermite electric matches developed by LANL can create simple sparks, hot slag, droplet, or flames as thermal-initiating outputs to ignite other incendiaries or explosives.[1] HazardsLike conventional thermite, super thermite usage is hazardous due to the extremely high temperatures produced and the extreme difficulty in smothering a reaction once initiated. Additionally, with nanothermites, composition and morphology are important variables for safety. For example, the variation of layer thickness in energetic nanolaminates can allow control of the reactivity of it.[7] The thermite reaction releases dangerous ultra-violet (UV) light requiring that the reaction not be viewed directly, or that special eye protection (for example, a welder's mask) be worn. In general, super thermites are extremely hazardous to handle because of it high sensitivity to electrostatic discharge (ESD) that is usually less than 15 micro joules. A method of reducing ignition sensitivity and improving handling safety of nanothermites has been developed which replaces the metal oxide nanoparticles with carbon nanofibres filled with manganese oxide (MnO2).[15] See alsoReferences
External links
9/11 Experiments by Jonathon ColeSome common lies and misconceptions about the properties and abilities of thermite and it's relation to the 9/11 WTC Demolitions: Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center CatastropheNiels H. Harrit, (Department of Chemistry, University of Copenhagen, Denmark)Jeffrey Farrer, (Department of Physics and Astronomy, Brigham Young University, Provo, UT 84602, USA) Steven E. Jones, (S&J Scientific Co., Provo, UT, 84606, USA) Kevin R. Ryan, (9/11 Working Group of Bloomington, Bloomington, IN 47401, USA) Frank M. Legge, (Logical Systems Consulting, Perth, Western Australia) Daniel Farnsworth, (Department of Physics and Astronomy, Brigham Young University, Provo, UT 84602, USA) Gregg Roberts6, (Architects & Engineers for 9/11 Truth, Berkeley, CA 94704, USA) James R. Gourley, (International Center for 9/11 Studies, Dallas, TX 75231, USA) Bradley R. Larsen (S&J Scientific Co., Provo, UT, 84606, USA)
INTRODUCTIONThe destruction of three skyscrapers (WTC 1, 2 and 7) on September 11, 2001 was an immensely tragic catastrophe that not only impacted thousands of people and families directly, due to injury and loss of life, but also provided the motivation for numerous expensive and radical changes in domestic and foreign policy. For these and other reasons, knowing what really happened that fateful day is of grave importance. A great deal of effort has been put forth by various government- sponsored and -funded investigations, which led, in large part, to the reports released by FEMA [1] and NIST [2]. Other studies of the destruction have been less well publicized but are no less important to the outstanding obligation that remains to the victims of that tragedy, to determine the whole truth of the events of that day [3] , [4] , [5] , [6] , [7] , [8] , [9]. A number of these studies have appropriately focused attention on the remaining physical material, and on available photographs and video footage, as sources of evidence still in public hands, relating to the method of destruction of the three skyscrapers. The collapses of the three tallest WTC buildings were remarkable for their completeness, their near free-fall speed [11] their striking radial symmetry [1], [12] and the surprisingly large volume of fine toxic dust [13] that was generated. In order to better understand these features of the destruction, the authors initiated an examination of this dust. In June 2007, Dr. Steven Jones observed distinctive bi-layered chips, with both a red and a gray layer, in a sample of the WTC dust. Initially, it was suspected these might be dried paint chips, but after closer inspection and testing, it was shown that this was not the case. Further testing was then performed on the red/gray chips in an attempt to ascertain their composition and properties. The authors also obtained and examined additional samples of WTC dust which had been collected by independent observers on, or very soon after, 9/11. All of the samples examined contained these very small, peculiar red/gray chips. Previous studies discussing observations of the WTC dust include reports by the RJ Lee Company [14], the U.S. Geological Survey (USGS) [15], McGee et al. [13] and Lioy et al. [16] Some of these studies confirmed the finding of iron-rich microspheres, which are also peculiar [5], [8], [11], [13], [14],[15] but the red/gray chips analyzed in this study have apparently not been discussed in previously published reports. It is worth emphasizing that one sample was collected about ten minutes after the collapse of the second Tower, so it cannot possibly have been contaminated by clean-up operations [17]. MATERIALS AND METHODS1. Provenance of the Samples Analyzed for this ReportIn a paper presented first online in autumn 2006 regarding anomalies observed in the World Trade Center destruction [6], a general request was issued for samples of the WTC dust. The expectation at that time was that a careful examination of the dust might yield evidence to support the hypothesis that explosive materials other than jet fuel caused the extraordinarily rapid and essentially total destruction of the WTC buildings. It was learned that a number of people had saved samples of the copious, dense dust, which spread and settled across Manhattan. Several of these people sent portions of their samples to members of this research group. This paper discusses four separate dust samples collected on or shortly after 9/11/2001. Each sample was found to contain red/gray chips. All four samples were originally collected by private citizens who lived in New York City at the time of the tragedy. These citizens came forward and provided samples for analysis in the public interest, allowing study of the 9/11 dust for whatever facts about the day might be learned from the dust. A map showing the locations where the four samples were collected is presented as Fig. (1). 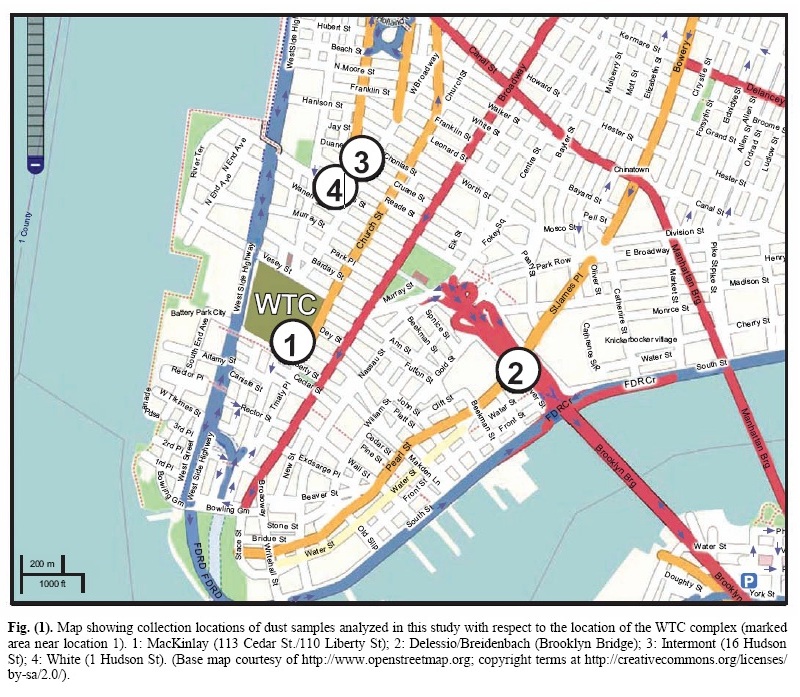
The earliest-collected sample came from Mr. Frank Delessio who, according to his videotaped testimony [17], was on the Manhattan side of the Brooklyn Bridge about the time the second tower, the North Tower, fell to the ground. He saw the tower fall and was enveloped by the resulting thick dust which settled throughout the area. He swept a handful of the dust from a rail on the pedestrian walkway near the end of the bridge, about ten minutes after the fall of the North Tower. He then went to visit his friend, Mr. Tom Breidenbach, carrying the dust in his hand, and the two of them discussed the dust and decided to save it in a plastic bag. On 11/15/2007, Breidenbach sent a portion of this dust to Dr. Jones for analysis. Breidenbach has also recorded his testimony about the collection of this dust sample on videotape [17]. Thus, the Delessio/Breidenbach sample was collected about ten minutes after the second tower collapsed. It was, therefore, definitely not contaminated by the steelcutting or clean-up operations at Ground Zero, which began later. Furthermore, it is not mixed with dust from WTC 7, which fell hours later. On the morning of 9/12/2001, Mr. Stephen White of New York City entered a room in his apartment on the 8th floor of 1 Hudson Street, about five blocks from the WTC. He found a layer of dust about an inch thick on a stack of folded laundry near a window which was open about 4 inches (10 cm). Evidently the open window had allowed a significant amount of dust from the WTC destruction the day before to enter the room and cover the laundry. He saved some of the dust and, on 2/02/2008, sent a sample directly to Dr. Jones for analysis. Another sample was collected from the apartment building at 16 Hudson Street by Mr. Jody Intermont at about 2 pm on 9/12/2001. Two small samples of this dust were simultaneously sent to Dr. Jones and to Kevin Ryan on 2/02/2008 for analysis. Intermont sent a signed affidavit with each sample verifying that he had personally collected the (nowsplit) sample; he wrote: “This dust, which came from the ‘collapsed’ World Trade Center Towers, was collected from my loft at the corner of Reade Street and Hudson Street on September 12, 2001. I give permission to use my name in connection to this evidence”. [Signed 31 January 2008 in the presence of a witness who also signed his name]. On the morning of 9/11/2001, Ms. Janette MacKinlay was in her fourth-floor apartment at 113 Cedar St./110 Liberty St. in New York City, across the street from the WTC plaza. As the South Tower collapsed, the flowing cloud of dust and debris caused windows of her apartment to break inward and dust filled her apartment. She escaped by quickly wrapping a wet towel around her head and exiting the building. The building was closed for entry for about a week. As soon as Ms. MacKinlay was allowed to re-enter her apartment, she did so and began cleaning up. There was a thick layer of dust on the floor. She collected some of it into a large sealable plastic bag for possible later use in an art piece. Ms. MacKinlay responded to the request in the 2006 paper by Dr. Jones by sending him a dust sample. In November 2006, Dr. Jones traveled to California to visit Ms. MacKinlay at her new location, and in the company of several witnesses collected a second sample of the WTC dust directly from her large plastic bag where the dust was stored. She has also sent samples directly to Dr. Jeffrey Farrer and Kevin Ryan. Results from their studies form part of this report. Another dust sample was collected by an individual from a window sill of a building on Potter Street in NYC. He has not given permission for his name to be disclosed, therefore his material is not included in this study. That sample, however, contained red/gray chips of the same general composition as the samples described here.
2. Chip Size, Isolation, and ExaminationFor clarification, the dust samples collected and sent to the authors by Ms. Janette MacKinlay will be sample 1; the sample collected by Mr. Frank Delassio, or the Delassio/ Breidenbach sample, will be sample 2; the sample collected by Mr. Jody Intermont will be sample 3; and the sample collected by Mr. Stephen White will be sample 4. The red/gray chips are attracted by a magnet, which facilitates collection and separation of the chips from the bulk of the dust. A small permanent magnet in its own plastic bag was used to attract and collect the chips from dust samples. The chips are typically small but readily discernible by eye due to their distinctive color. They are of variable size with major dimensions of roughly 0.2 to 3 mm. Thicknesses vary from roughly 10 to 100 microns for each layer (red and gray). Samples of WTC dust from these and other collectors have been sent directly from collectors to various scientists (including some not on this research team) who have also found such red/gray chips in the dust from the World Trade Center destruction. An FEI XL30-SFEG scanning electron microscope (SEM) was used to perform secondary-electron (SE) imaging and backscattered electron (BSE) imaging. The SE imaging was used to look at the surface topography and porosity of the red/gray chips, while the BSE imaging was used to distinguish variations in average atomic number, Z. The microscope was also equipped with an EDAX X-ray energy dispersive spectrometry (XEDS) system. The XEDS system uses a silicon detector (SiLi) with resolution better than 135 eV. The spectrum resolution was set to 10 eV per channel. Operating conditions for the acquired XEDS spectra were 20 keV beam energy (unless otherwise specified) and 40-120 second acquisition time (livetime). XEDS maps were acquired using the same system at a beam energy of 10 keV. For general surface analysis in the SEM, dust samples were mounted to carbon conductive tabs. The samples were left unwashed and uncoated unless otherwise specified. In order to more closely observe the characteristics of the red and gray layers, and to eliminate the possibility of surface contamination from other dust particles, several red/gray chips from each of the four WTC dust samples were fractured. The clean, cross-section surfaces were then studied by BSE imaging and XEDS. 10 The Open Chemical Physics Journal, 2009, Volume 2 Harrit et al. Some samples were also tested in a differential scanning calorimeter (Netzsch DSC 404C) to measure heat flow into or out of the red/gray chips. The DSC tests were conducted with a linear heating rate of 10 °C per minute up to a temperature of 700 °C. During heating, the samples were contained in alumina pans and air was allowed to flow at 55 milliliters per minute during the heating. The plots were generated by acquiring data points at a rate of 20 points per °C or 200 points per minute. The equipment was calibrated to display the data in watts per gram. The plots were set to display positive heat flow out of the sample such that exothermic behavior of the sample would yield a peak and endothermic behavior a trough. The dust samples were also examined by visible-light microscopy (VLM) through a Nikon Epiphot 200 stereomicroscope, an Olympus BX60 stereomicroscope and a Nikon Labophot microscope and camera. RESULTS1. Characterization of the Red/Gray ChipsRed/gray chips were found in all of the dust samples collected. An analysis of the chips was performed to assess the similarity of the chips and to determine the chemistry and materials that make up the chips. Fig. (2) displays photomicrographs of red/gray chips from each of the four WTC dust samples. Note the scale marker in each image as they were acquired at different magnifications. At approximately 2.5 mm in length, the chip in Fig. (2a) was one of the larger chips collected. The mass of this chip was approximately 0.7 mg. All of the chips used in the study had a gray layer and a red layer and were attracted by a magnet. The inset image in Fig. (2d) shows the chip in cross section, which reveals the gray layer. The gray layer is also partially visible in Fig. (2b). Similarities between the samples are already evident from these photographs. Fig. (3) shows three images for comparison of views of the same set of chips using different methods. Fig. (3a) is a VLM photomicrograph of a group of particles, which shows the red material and in some cases the adhering gray material. Fig. (3b, c) are, respectively, a secondary electron (SE) image and a backscattered electron (BSE) image of the same group of particles, using a scanning electron microscope (SEM) without a conductive coating over the sample. It can be seen in the SE image that the red layer of the particles has very bright regions caused by a slight accumulation of charge under the electron beam, owing to the relatively poor conductivity of the red layer (see Discussion section). The BSE image shows the red layer darker than the gray layer, 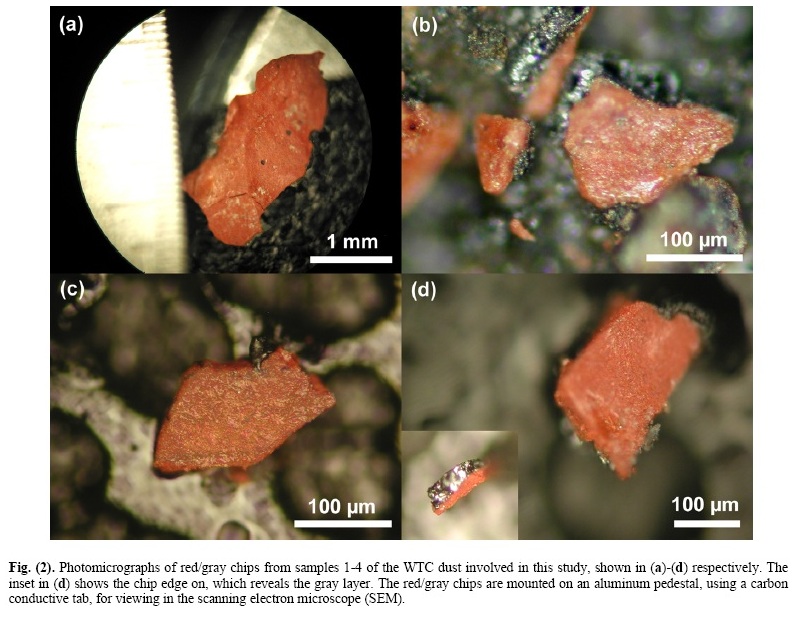


Fig. (4). Higher magnification BSE image of one of the chips in previous image. The red layer appears darker and is on top of the gray layer. 
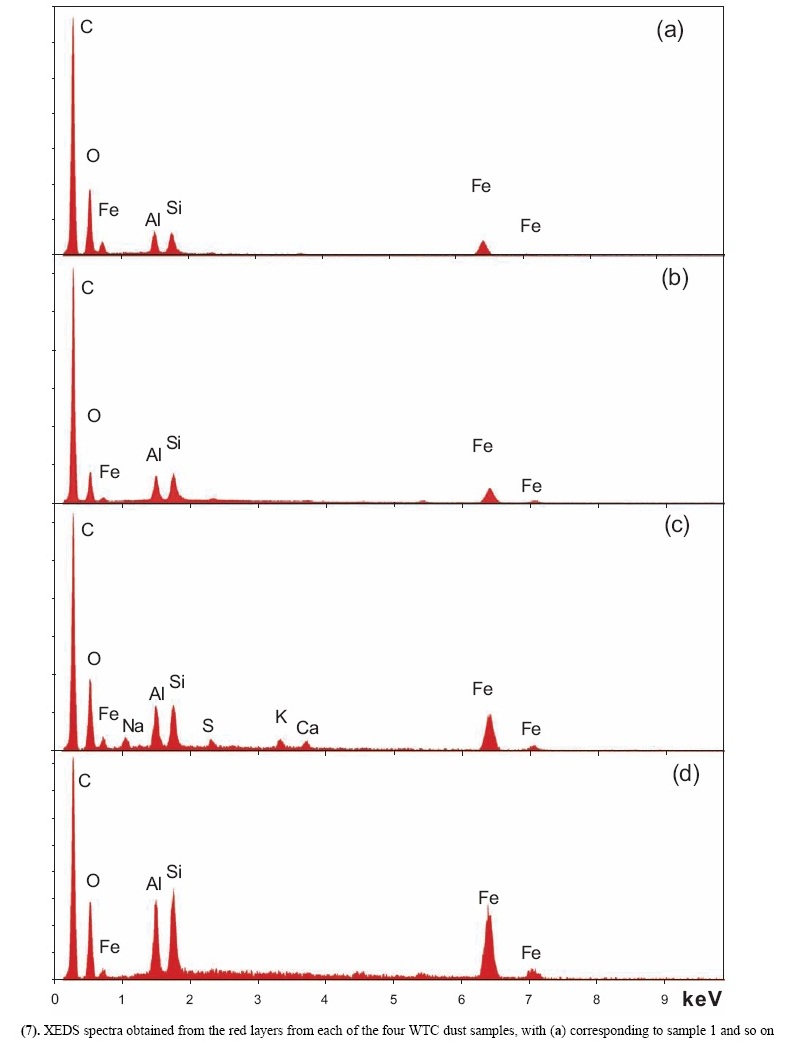
X-ray energy-dispersive spectroscopy (XEDS) analyses of both the red and gray layers from cross sections prepared from the four dust samples were performed and representative spectra are shown in Figs. (6, 7). The four spectra in Fig. (6) indicate that the gray layers are consistently characterized by high iron and oxygen content including a smaller amount of carbon. The chemical signatures found in the red layers are also quite consistent (Fig. 7), each showing the presence of aluminum (Al), silicon (Si), iron (Fe) and oxygen (O), and a significant carbon (C) peak as well. At still higher magnifications, BSE imaging of the red layer illustrates the similarity between the different dust samples. BSE images of small but representative portions of each red-layer cross section are shown in Fig. (8). The results indicate that the small particles with very high BSE intensity (brightness) are consistently 100 nm in size and have a faceted appearance. These bright particles are seen intermixed with plate-like particles that have intermediate BSE intensity and are approximately 40 nm thick and up to about 1 micron across. Furthermore, by comparing the BSE image in Fig. (8a) to the SE image in Fig. (9), it can be seen that all of the particles are embedded in an unstructured matrix which gives a dark BSE intensity. XEDS maps of the cross-section surface of the red layer were acquired at a beam energy of 10 kV. The acquisition area of the maps is shown by the BSE image in Fig. (10a). The XEDS maps, several of which are shown in Fig. (10b-f), indicate by color, the degree to which the particular element is present at or near the surface from point to point across the area. The results indicate that the smaller particles with very bright BSE intensity are associated with the regions of high Fe and O. The plate-like particles with intermediate BSE intensity appear to be associated with the regions of high Al and Si. The O map (d) also indicates oxygen present, to a lesser degree, in the location of the Al and Si. However, it is inconclusive from these data whether the O is associated with Si or Al or both. The carbon map appears less definitive, that is, it does not appear to be associated with a particular particle or group of particles, but rather with the matrix material. In order to learn more from these findings, a focused electron beam was placed directly onto the different particles, and the XEDS data were collected. By placing the beam on a cluster of plate-like particles, the spectrum in Fig. (11a) was generated. The spectrum in Fig. (11b) was acquired 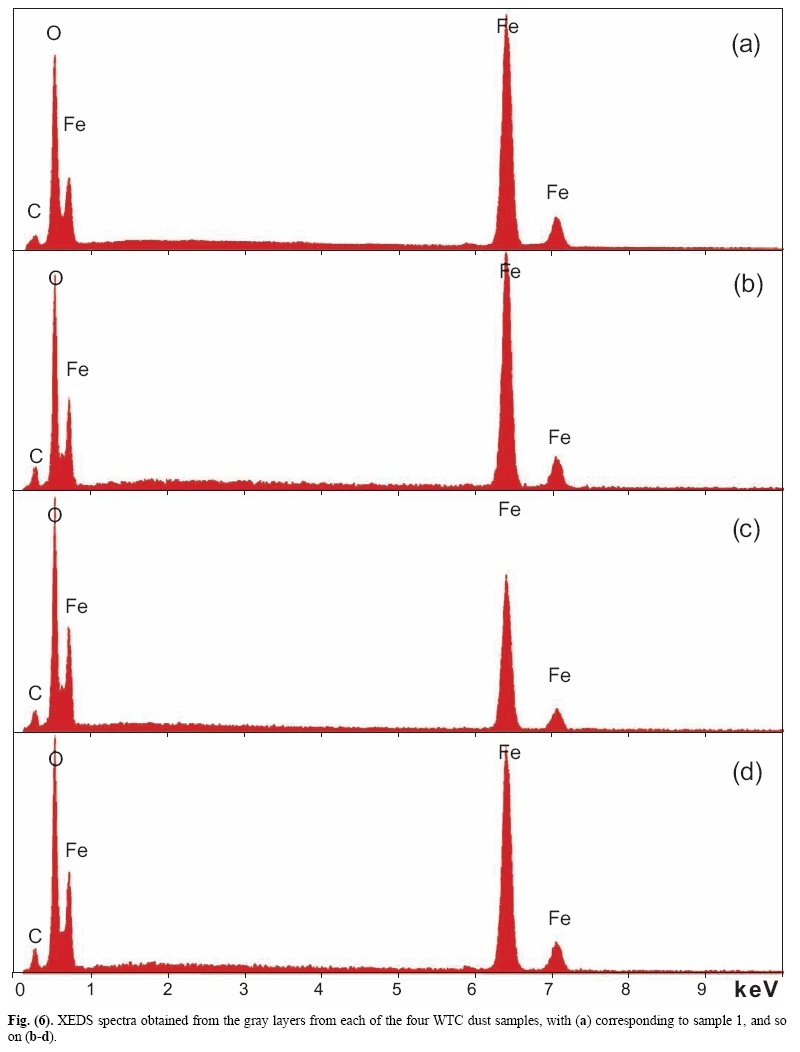
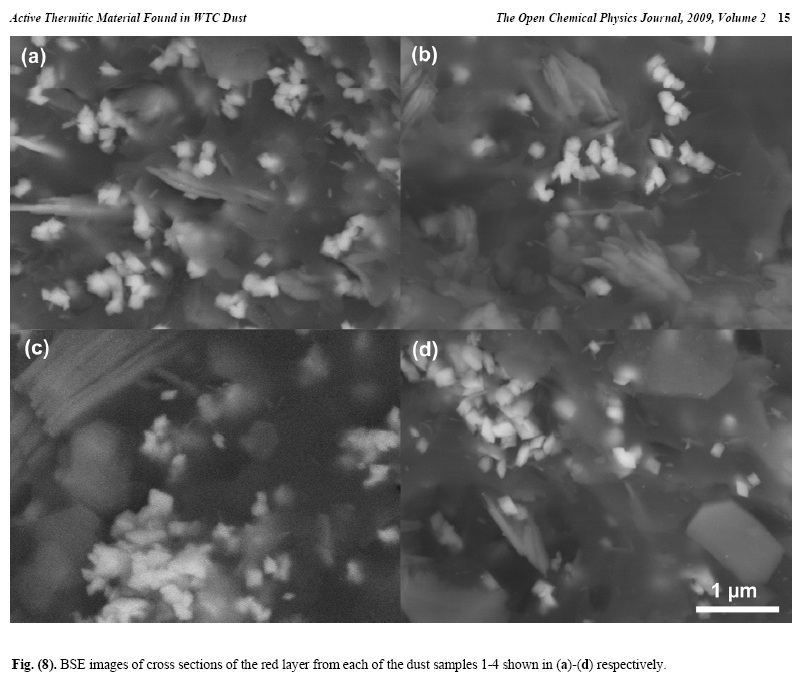
The consistently rhombic-shaped, faceted appearance of the iron-rich grains strongly suggests that they are crystalline. From these data, it is determined that the red/gray chips from different WTC dust samples are extremely similar in their chemical and structural makeup. It is also shown that within the red layer there is an intimate mixing of the Fe-rich grains and Al/Si plate-like particles and that these particles are embedded in a carbon-rich matrix. 2. Test Using Methyl Ethyl Ketone SolventBy employing some means to separate the different components of the material, the chemical compositions of the different particles in the red layer were more accurately The rest of this paper can be found at http://www.bentham.org/open/tocpj/articles/V002/7TOCPJ.pdf |